Mimicking
Bone - Chemical and Physical Challenges
Sophie
C. Cox (WMG,
University of Warwick)
Abstract
It
is known that chemical and physical features of bone contribute to its
functionality, reactivity and mechanical performance. This article
presents a
summary of previously published studies conducted by the author with
the aim of
fabricating a synthetic structure, referred to as a scaffold, which
both
chemically and physically emulates the intricate structure of bone.
Novel work
aimed at improving the understanding of the synthesis of a ceramic
biomaterial,
namely hydroxyapatite, that is chemically similar to bone mineral is
discussed.
A case study involving the manufacture of porous scaffolds by 3D
printing is
also presented. In summary, this article highlights a number of
on-going
challenges that multidisciplinary tissue engineers aim to solve to get
one-step
closer to mimicking bone, which clinically could improve the quality of
life
for millions of people worldwide.
Keywords:
Bone
tissue engineering;
Scaffolds; Hydroxyapatite; Synthesis; Characterisation
Introduction
Bones
perform several vital
functions within the body, primarily structural support and protection
of
bodily organs. The ability of bone to self-repair and remodel to meet
varying
mechanical demands makes it a unique structural composite material (Chamay
and Tschantz 1972; You
et al.
2010).
Bone also serves as (a) an attachment
site for muscles to enable limb movement and joint mobility, (b) a
reservoir
for minerals (e.g. calcium and phosphorous), and (c) the primary site
for the
synthesis of blood cells.
The
capacity of bone to
function healthily can be affected by pathological conditions,
diseases, and it
is also well known that bone degenerates with age (Ritchie et
al. 2006).
Furthermore, the ability of bone to
self-repair is limited by what is known as the ‘critical size
defect’, defined
by Schmitz and Hollinger as “the smallest intraosseous wound in a
particular
bone and species of animal that will not heal spontaneously during the
lifetime
of the animal” (1986).
Major
alterations in bone
structure due to injury or disease can lead to discomfort and a reduced
quality
of life. Furthermore, defects outside of the limitations of natural
self-repair
may require surgical intervention, thus creating a demand for
appropriate
clinical strategies.
The
highly organised and
complex structure of bone, however, means this clinical need presents
an on-going
medical challenge. Traditionally bone grafts (BGs) and most commonly
autografts
– tissue harvested from another location in the patient’s
body – have been used
to fill or heal such defects. Despite being the ‘gold
standard’, there are
several disadvantages of autologous BGs, including painful harvesting
surgery,
limited supply, and long recovery times. These shortcomings have driven
the
biomedical research community to investigate alternative solutions that
incorporate the use of synthetic biomaterials.
Specifically,
an alternative
strategy to traditional approaches is to create a temporary surrogate
structure, which guides and encourages tissue regeneration. In order
for such a
strategy to be successful, it is necessary to combine expertise of
cells,
biochemical factors, and biomaterial science. This interdisciplinary
field of
research is known as tissue engineering and the structural component of
this
strategy is referred to as a ‘scaffold’.
Ideally,
a scaffold should
emulate the chemical and physical structure of the native tissue, thus
it is
crucial that an understanding of the properties that infer the
functionality of
bone is developed. Much attention has been given to calcium phosphate
(CaP)
based biomaterials since generally they are chemically similar to bone
mineral.
In particular, hydroxyapatite (Ca10(PO4)6(OH)2
- HA) has been shown to exhibit a comparable crystal structure (Posner
1969, Cazalbou et
al. 2004).
This chemical likeness and the natural
biological response that is elicited upon implantation is a key
advantage of HA
in comparison to other biomaterials commonly used in orthopaedic
applications,
such as metals (e.g. Ti6Al4V), polymers (e.g. HDPE) or bioglasses (e.g.
45S5). The
Food and Drug Association (FDA), a USA agency that protects public
health
through supervision and regulation, has extensive guidelines for the in-vivo use of HA in the form of, for
example, metallic implant coatings and void fillers.
This
material, despite its
promising features is known to exhibit poor resorbability and
reactivity under
physiological conditions compared to natural bone mineral due to its
high
stability (Cengiz et al.
2008).
Substitution of trace elements that
naturally occur in bone (for example Mg, Sr, Zn) and the use of
nanosized (10-9m)
HA (nHA) have been shown to enhance the solubility, reactivity and
response of
bone cells to this synthetic material in-vitro
and in-vivo (Webster et al.
2001; Leventouri 2006; Boanini et al.
2010).
It is, however, important to note,
that any changes to chemical composition must seek further FDA
approval, which
is a lengthy and costly process. Much effort has been focused on the
synthesis
of this bioceramic due to the potential applications of HA as a bone
replacement material (Elliott
1994; Raynaud et al.
2002; Bose and Saha 2003; Landi et al.
2008).
The majority of such studies, however,
are restricted to the preparation and structural investigation of HA
without an
evaluation of biological performance (Xue et al.
2006).
Lack of biological
testing is likely due to it being an expensive and time-consuming
process. In
addition, it may also be true, in some cases, that this is combined
with a lack
of appreciation of the effects of physicochemical alterations to the
biological
performance of HA.
Numerous
authors have
reported the fabrication of pure or composite HA scaffolds using a
variety of
techniques (Hutmacher
2000; Macchetta et
al. 2009).
In recent decades focus has been
directed to the use of additive layer manufacturing (ALM) systems to
produce
such constructs layer-by-layer since they can be user defined, which
inherently
improves reproducibility and enables the creation of patient-specific
products.
It is relatively common that commercially purchased HA is used as a
precursor
material and as such the motivation of such studies is narrowed to the
influence of physical attributes. That is despite the fact that the
reactivity
of bone mineral is largely determined by its composition and crystal
structure,
which in turn is determined by the synthesis method and reaction
conditions (Cazalbou et
al. 2004).
This
article presents an
overview of the author’s research to date while highlighting
other key works
within the field. The studies presented are focused on developing an
understanding of how the conditions used during aqueous precipitation
(AP) of
HA and 3D printing (3DP) influence critical scaffold properties.
Ultimately, the
aim of this article is to unearth chemical and physical challenges
involved in
mimicking bone and discuss the next steps of the author’s work as
well as the
field of biomaterials.
Chemical
Challenges
The
largest component of bone by weight
is the mineral phase (65 – 70wt%), which can be described as a
non-stoichiometric carbonated multi-substituted apatite that exhibits a
similar
crystal structure to HA. There
are numerous
conventional techniques that have been used to synthesise synthetic HA.
A
review of such methods is outside the remit of this article,
however
a
comprehensive overview is presented in the author’s thesis (Cox
2013).
This article
is focused on synthesising HA via aqueous precipitation (AP), which is
a
popular method due
to the use of relatively cheap raw materials and low temperatures
resulting in
minimal operating costs. AP reactions, however, cannot be deemed as
trivial due
to the simultaneous occurrence of crystal nucleation, growth, as well
as
coarsening and/or agglomeration. These underlying scientific mechanisms
are not
easy to differentiate and as a result reproducibility as well as the
control of
particle flocculation remain common factors for improvement (Narasaraju
and Phebe 1996,
Suchanek
and Yoshimura 1998,
Phillips et al. 2003).
The sensitivity
of phosphates and the need to fine tune the experimental conditions
(e.g. pH,
temperature) of AP reactions is reflected in the literature by the
ranging
phase purity, particle morphologies as well as sizes, crystallinity and
thermal
stability. Despite the shortcomings of AP methods, the potential to
produce HA
containing various ionic substitutions and its high scalability make it
an attractive
methodology, particularly for industrial scale production (Boanini et al.
2010).
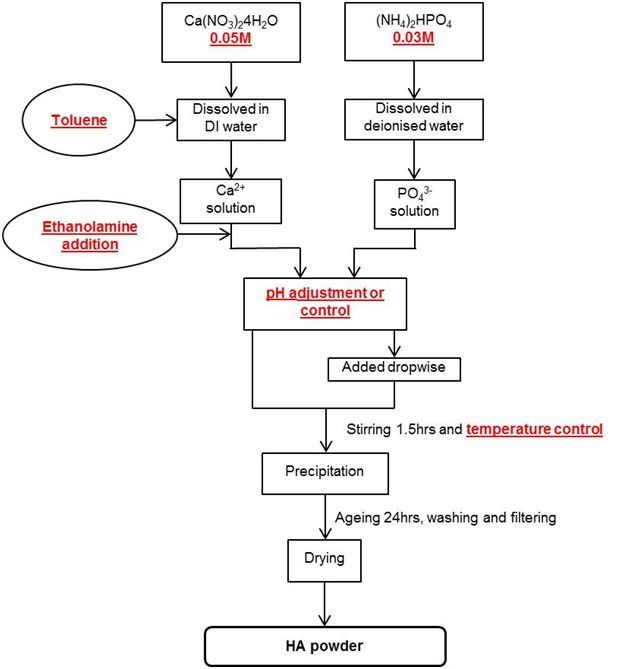
The
AP method used by the author to synthesise samples A – G (Table
1) is outlined in Figure 1, for full details please refer to the
referenced
publications.
Figure
1: Flowchart illustrating the aqueous precipitation method used by Cox et al. to synthesise hydroxyapatite with
varied conditions highlighted in red
In
spite of the widespread
use of AP methods and the range of reaction conditions reported in the
literature, few authors have systematically investigated the
relationship
between such conditions and biological performance. Novel work by Cox et al. showed through the use of in-vitro
assays utilising MC3T3
osteoblast precursor cells, derived from mice, that changing the pH,
temperature and solvent used during the AP of HA can significantly
affect the
degree of proliferation on such substrates (Cox
2013; Cox et al.
2014).
Table 1 summarises
the conditions that HA samples were precipitated under and results of
the
performed in-vitro assays.
Fluorescence micrographs revealed that not maintaining the pH at 11
during
synthesis (Sample A) resulted in dead (red) cells after 1 day of
culture,
illustrating the critical importance of the value and control of this
parameter
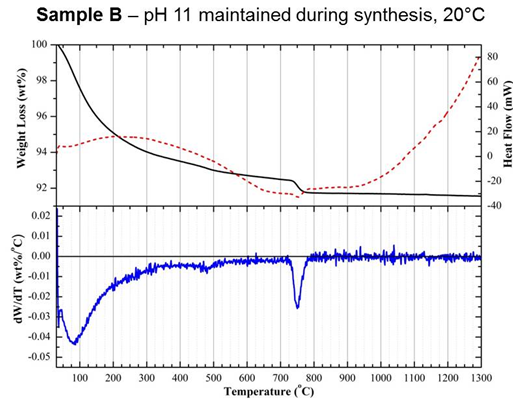
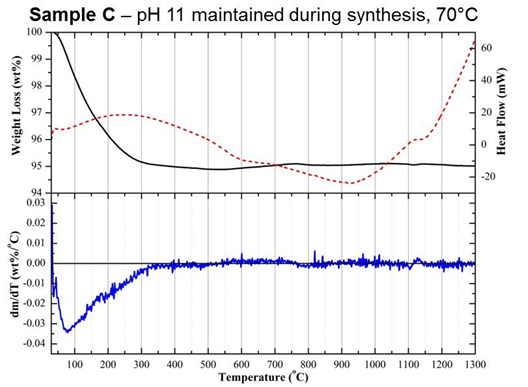
(Figure 2). A comparison of samples B and C, prepared under the same pH
conditions but at 20 and 70°C, respectively, highlights that
fine-tuning of AP
reactions is vital to ensure a non-cytotoxic HA substrate is produced
(Figure
2). Both of these differences in biological outcomes were attributed to
subtle
changes in physicochemical properties, which were identified through
the
combined use of a range of material characterisation methods, including
surface
area analysis, zeta potential, as well as simultaneous differential
thermal
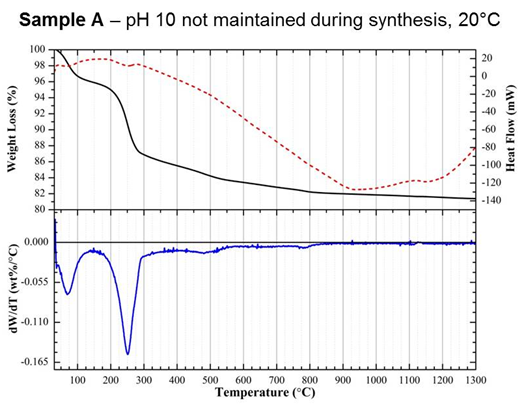
analysis (DTA) and thermogravimetry (TGA). In particular,
differentiation of
TGA curves (DTG) collected up to 1300°C
revealed
a significant change in the rate of weight loss for sample A between
200 and
300°C,
which
was not seen in samples B or C (Figure 3). Weight loss within this
region was
attributed to the dehydration of an acidic CaP phase, dicalcium
phosphate
dihydrate (CaHPO4·2H2O), otherwise known
as brushite.
Samples A and C were both shown to exhibit positive zeta potentials
compared
with the varying degrees of negative values measured for samples that
were
shown to be non-cytotoxic using a dead/live assay, such as sample B
(Table
1). These positive values were attributed to the presence of secondary
acidic
CaP phosphates, which are proposed to have been released during culture
resulting in cell death.
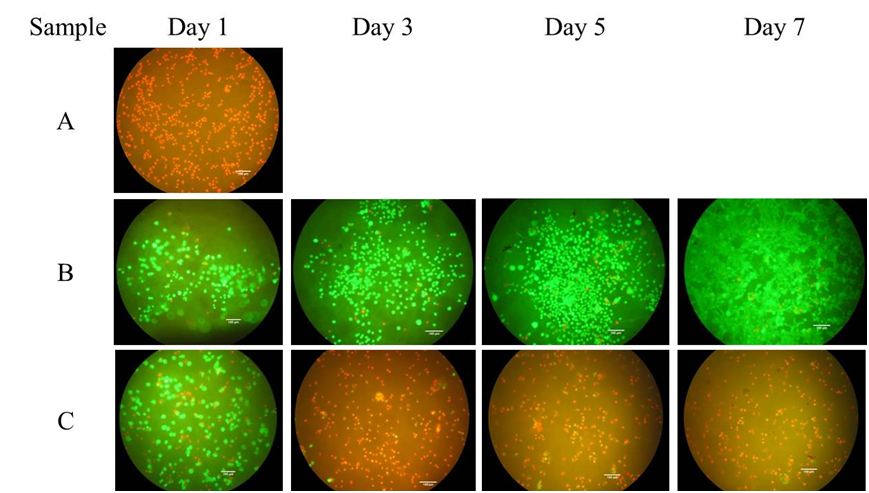
Figure
2: Fluorescence
micrographs from a dead/live assay illustrating the critical influence
of pH and temperature on the viability of MC3T3 cells from 1 to 7 days
of culture (red = dead; green = live) (Cox
2013)
In
addition to pH and
temperature, the author has also previously investigated the effect of
changing
the solvent in which the HA crystals are precipitated. A proliferation
rate of
194% between 1 and 7 days of culture of MC3T3 cells seeded on HA
substrates
precipitated in a mixed Toluene and deionised (DI) water solvent system
was
calculated from results of an in-vitro
MTT assay (Table 1). This was compared with a 82% increase in cell
number on HA
prepared in the same manner but in a pure DI water system (Cox
2013).
This significant
increase in cell number was attributed to a reduction in the dielectric
constant of the solvent system, as a result of using Toluene instead of
polarised DI water, reducing the degree of crystallinity and surface
charge as
well as increasing the surface area of precipitated HA particles (Table
1).
The
hexagonal crystal
structure of HA enables partial or total replacement of ions, and in
bone
apatite numerous biologically relevant ionic substitutions occur.
Divalent
cations (e.g. Mg2+) and monovalent cations (e.g. K+)
can
substitute for Ca2+, and anions such as fluoride (F-)
or
chloride (Cl-) may substitute for OH- groups.
Furthermore, some ions such as carbonates can substitute for OH-,
PO43-
or
both, which are referred to as A, B and AB type substitutions,
respectively (Cazalbou et
al. 2004).
Therefore, an
appropriate formula for bone apatite can be expressed as (Ca, X)10(PO4,
CO3, Y)6(OH, Z)2 with X as
substituting cations,
and Y and Z being the substituting anions, with the indices 10, 6 and 2
changing according to the degree of stoichiometry (Weiner
and Wagner 1998).
In an effort to
emulate the higher reactivity of bone mineral, a number of biomedical
groups
concerned with CaPs have investigated substituting naturally occurring
ions
into synthetic HA (Capuccini et
al. 2008; Landi et al.
2008; Bohner
2009).
A number of reports have confirmed
that substitution of Sr, Mg or Zn can influence the crystallinity,
solubility,
surface charge, and physiological dissolution rate of synthetic
CaPs (Boanini et al.
2010).
Each of these divalent cations are known
to play important roles in the biological responses of bone cells.
Despite the
synthesis of substituted HA attracting much interest, to the best of
the
author’s knowledge Sr, Mg or Zn substituted HA prepared under the
same
experimental conditions has not previously been reported. As
highlighted above changes
in reaction conditions, such as pH, can greatly influence the physical,
chemical, and crystal structure of precipitated apatite, which makes it
difficult to determine the sole influence of substituents when
comparing
literature since variations in synthesis conditions, particularly pH
and
temperature, are common. Hence it is proposed that a comparison of HA
doped
with Sr, Mg or Zn prepared under the same experimental conditions is a
more
accurate assessment of the individual influence of these cations. Such
a study
was previously published by the author and concluded that doping HA
with 10mol%
Mg or 2mol% Zn enhanced the degree of cellular proliferation over a 7
day
period compared with pure HA (Cox et al.
2014).
The significantly
higher proliferative rates calculated for Mg- and Zn-HA were attributed
to the
altered composition as a result of incorporation of dopants into the
lattice
structure and increased surface areas of particles (Table 1).
In
summary, the author’s previously
published work that is discussed provides evidence that cell
proliferation may
be positively influenced by maintaining a pH level of 11 during
synthesis,
precipitating HA in a mixed Toluene and DI water system, and doping HA
with 10mol%
Mg or 2mol% Zn. Generally, the selection of a synthesis method and
conditions
to produce HA cannot be deemed a trivial step in addressing the
chemical
challenge of mimicking bone mineral (Cox et al.
2014).
In the words of
Drouet, ‘all that glitters is not gold… all that is white
is not apatite
either’ (Drouet
2013).
Physical
Challenges
Bone
exhibits a
heterogeneous and anisotropic structure that comprises different
components at
a range of length scales. Macroscopically bone is distinguished into
cortical,
otherwise known as compact, and cancellous, also referred to as
trabecular or
spongy bone. Cortical and cancellous bone can be easily distinguished
by their
degree of porosity: 4 - 28%, and 40 - 95%, respectively (Gibson
1985).
The denser
structure of cortical bone forms the outer region of all types of bone,
the
diaphysis (shaft) of long bones, and flat bones providing protection
and
support for the inner regions. In contrast, cancellous bone exhibits
macro-sized pores, filled with bone marrow, which is found in the
centre of all
bones. The intricate organisation of bone confers its functionality,
however,
for biomedical scientists this precise structural arrangement presents
a number
of challenges when trying to produce a scaffold that mimics its
porosity and
pore sizes, interconnectivity, topography, and mechanical strength.
Broadly,
scaffold
fabrication methods can be grouped as conventional or ALM techniques.
In
general, the fabrication method should adhere to: (1) does not
adversely affect
the chemical composition, mechanical properties or cytocompatibility of
the
material, (2) the technique should be accurate so pore size and
morphology can
be defined by the user, and (3) minimal variation in physical form
between
batches (i.e. consistency) (Leong et al.
2003).
Scaffolds of complex shapes with a
range of process dependent porosities from 30 - 98% can be produced by
conventional techniques, such as freeze-drying or solvent casting. In
contrast
to ALM technologies, conventional processes require small capital input
but
typically scaffolds made using these techniques perform poorly
mechanically in
comparison to native bone. Reproducibility is another issue associated
with
these methods due to the inability to precisely control scaffold
characteristics, such as pore size, pore interconnectivity and spatial
distribution of pores. Conventionally fabricated constructs commonly
fail to
meet the demand to create highly porous networks necessary for cell
growth, flow
of nutrients and metabolic waste (Hutmacher
2000).
Often, these techniques also require
the use of organic solvents, such as chloroform, which if any residues
remain
in the structure may be toxic and/or carcinogenic to cells.
There
are a variety of ALM
systems based on computer aided design (CAD) and manufacture (CAM).
Such
techniques were first used for biomedical applications in the 1990s and
can be
categorised into three groups: (1) laser (e.g. stereolithography), (2)
print or
‘ink’ (e.g. 3D printing) and (3) nozzle systems (e.g. fused
deposition
modelling). A comprehensive review of ALM techniques for the
fabrication of
bone scaffolds is presented by Hollister (2005).
Generally, ALM techniques can be used
to accurately fabricate parts of complex designs and near net shape
processing
minimises material waste. Furthermore, data generated from a scan (CT
or MRI)
of the patient may also be used as a template allowing the manufacture
of
customised implants, meaning desired levels of hierarchical complexity
can be
built into the part, which is advantageous when trying to mimic the
intricate
physical structure of bone. Due to the higher level of structural
control it is
possible to produce scaffolds via ALM techniques with superior
mechanical
properties in comparison to conventionally manufactured
counterparts (Cox
2013).
The
author’s work is focused
on the use of an ALM technique called 3D printing (3DP), which is a
powder
based technology that involves layers of the stock material being
bonded
together by an appropriate liquid binder that is propelled onto the
powder bed
from a printer head. This process is much the same as what happens in
an
ink-jet printer but it is repeated layer-by-layer until the final 3D
part is formed.
Any unbound material may act as a support during the building process,
however,
this material must be removed after printing. In addition to exhibiting
the
inherent advantages of ALM systems, such as geometry control, 3DP
creates parts
with a rough surface. This is particularly advantageous for a bone
tissue
scaffold since the pits and troughs provide fixation points for cells
to adhere
to when it is implanted. Furthermore, the imperfect packing of powder
particles
results in small micropores within the solid structure, which may
facilitate
cell in-growth, vascularisation, and fluid flow.
For
the 3DP process to work,
it is essential that the powdered stock material has an ability to flow
to
enable adequate recoating of the part by the counter-rotating roller
during
layer-based manufacture. Particle size, morphology, and density have
been
reported to be critical factors in determining powder flowability (Butscher et
al. 2012).
A high level of flowability
contributes towards an improvement in the resolution of the final part
and vice
versa. However, if the flowability is too high the powder bed can
become
unstable. Wettability of the powder particles by the binder solution is
another
crucial factor as it influences both resolution and mechanical strength
of the
3D printed part. There are a number of parameters that influence powder
wetting,
including binder viscosity, topography of the powder bed surface (which
itself
is dependent on particle shape and size), and any chemical reactions
between
the binder and powder (Sachs et al.
1993).
Numerous
authors have
reported the fabrication of porous scaffolds by 3DP (Bose et al.
2013, Travitzky et
al. 2014).
Pure CaP powders,
for example α- and β-tricalcium phosphate (TCP) (Butscher et
al. 2012),
tetracalcium phosphate (Khalyfa et al.
2007),
and HA (Roy et al.
2003)
as well as composites of CaPs mixed
with organic polymers, such as poly(L-lactide-co-glycolide)-copolymer
(PLGA) (Roy et al.
2003),
have been utilised as stock materials.
Material combinations that require the use of organic solvents as a
binder, for
example PLGA and β-TCP binded with chloroform (Roy et al.
2003),
have an inherent disadvantage as
complete removal of the solvent is rather difficult due to the inherent
porosity of 3D printed structures.
The
feasibility of
fabricating 3D porous scaffolds from powder compositions of HA and
polyvinyl
alcohol (PVOH), suitable for use as a component of the tissue
engineering
strategy has been reported by the author (Cox
2013).
In short, to
manufacture constructs purchased PVOH and HA powders were mixed
together,
compacted into the powder bed of a ZPrinter 310 (ZCorp, USA) and bound
together
using a commercially available binder (Zb90, ZCorp, USA) using a user
defined
layer thickness of 0.1mm. Post-printing, scaffolds were left to dry for
1hr
before removing from the build bed, de-powdered using compressed air,
dried in
either a furnace or vacuum oven at 60°C
for either 2 or 6hrs, and sintered at 1300°C.
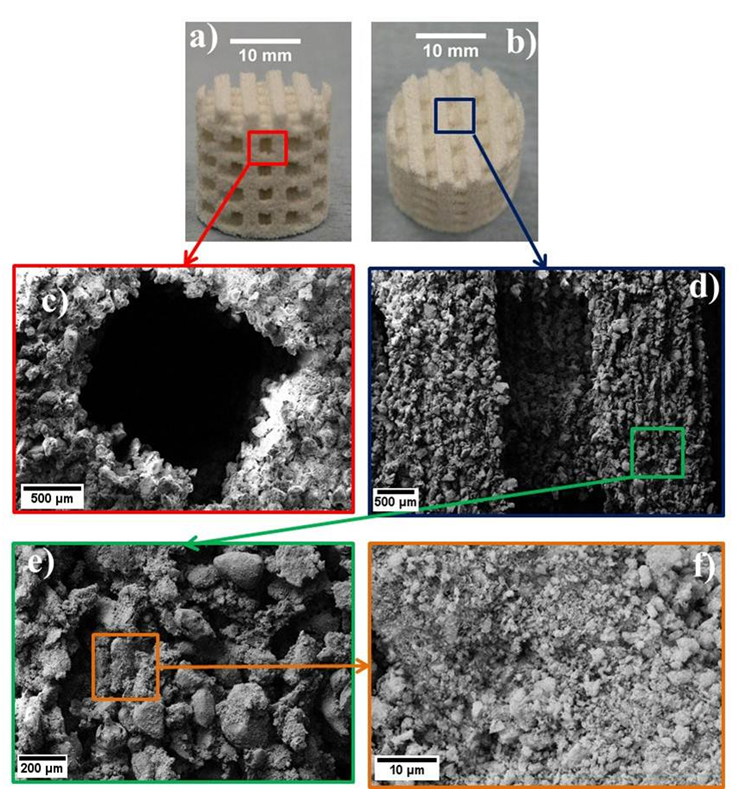
This
study highlighted the potential of 3DP to produce constructs that
exhibit key
structural criteria, such as surface roughness and porosity (Figure 4),
which
are known to be vital in determining the success of bone tissue
scaffolds.
Figures 4c and d illustrate that designed pore channels and struts were
accurately reproduced, this is important since the dimensions of these
features
were selected to enable cell migration and vascularisation. It is also
significant that topographical features from the mm (the designed
pores) to µm (surface
topography) are present, since a range of features are required for
different
biochemical effects/functions. For example, protein interactions
benefit from
micron sized features, cellular development is facilitated by pores
1-20µm,
bone in growth is improved by topographical pores 100-1000µm and
implant
functionality enhanced by pores >1000µm in size (Sanchez-Salcedo
et al.2008).
Figure
4: 3D printed scaffolds manufactured from 50HA:50PVOH powders
a)
Side view b) Top view, c) Designed pore channel, d) Scaffold strut, e)
and f)
Topography of scaffold surface (Cox
2013)
Characterisation
of powder flowability,
assessed using a funnel flow method, strongly correlated with observed
printability and can be deemed a vital prerequisite property since it
influenced recoating of the build bed, which ultimately determined
several
critical physical criteria such as mechanical strength, microstructure,
and
porosity. Scaffolds produced from less flowable precursors (i.e.
60HA:40PVOH)
were shown to be substantially weaker in compression and this was
attributed to
insufficient bonding between layers.Characterisation of green bodies
(i.e.
unsintered scaffolds) provided valuable information that facilitated an
understanding of the shrinkage behaviour observed as a result of
sintering to
1300°C.
Significant differences in the size of micropores were observed between
green
scaffolds printed along the X and Y-axes, which resulted in variation
of
mechanical strength, and influenced the effectiveness of the removal of
degradation products of PVOH during sintering. Simply the more voids,
or pores,
within the structure the weaker it is. Drying of green bodies after
printing
was shown to improve the compressive strength of scaffolds by up to
350%; this
was attributed to the shrinkage of micropores. Compression tests,
performed on
a 5800R 100kN static tester (Instron, UK) with 1kN load cell at a cross
head
speed of 10mm/min, also highlighted that interlayer bonding was
critical to bulk
strength and when parts were loaded perpendicular to the direction of
printing
(i.e. parallel to the boundaries of interlayer bonding) catastrophic
failure (i.e.
no or little plastic deformation) occurred. Highlighting that printing
direction is a critical consideration to implant design. A maximum
average
compressive strength of 0.88±0.02MPa was exhibited by 50wt% HA
green parts
printed along the Y-axis and dried for 6hrs in a vacuum oven at 60°C,
which is above the lower
value of 0.15MPa reported for cancellous bone (Cox
2013).
Conclusions
Bone
exhibits a highly
intricate physical structure and complex chemical composition, which
presents
the multidisciplinary field of tissue engineering with a number of
challenges
when trying to produce synthetic scaffolds that emulate this remarkable
composite. HA is a promising biomaterial for use in strategies to
replace bone
but our current understanding of this deceptive ceramic is not yet
complete as
highlighted by the author’s previous work, which is discussed in
this article. The
results of the synthesis studies referred to strongly advocate that pH
should
be maintained at 11 during preparation to ensure that a non-cytotoxic
precipitate is formed. Furthermore, the potential advantages of using
other
solvent systems to water was highlighted by the promising cell studies
conducted on HA synthesised in a mixed Toluene and water system.
Overall, the
author’s work in this area to date suggests that synthesis
conditions must be
carefully considered since they may have significant effects on cell
proliferation in-vitro.
The
3D printed scaffolds
illustrated in this article exhibit promising features for use in bone
tissue
engineering; rough topography for cell adhesion, compressive strength
values
comparable to cancellous bone, and an accurate replication of a user
defined
interconnected porous structure. Experimental work highlighted the
importance
of precursor characterisation and the advantages of printing along the
same
axis to part loading; in this case the Y-axis, as well as drying the
parts in a
vacuum oven for 6 hrs.
When
considering the major
advantage of ALM techniques to produce bone tissue scaffolds; the
ability to physically
tailor the construct, why should this idea not be combined with a
chemically
tailored stock material? The major shortcoming of the presented
research is the
challenges that the presented studies address have been achieved by
focusing
individually on either chemical or physical features. When looking at
the
author’s work as a whole it demonstrates the importance of
investigating
processing conditions so that techniques may be better understood and
ultimately this may translate to improved patient outcomes. Overall,
the
results presented are the foundation of further work, which is being
conducted
to combine the outcomes of the aqueous precipitation and 3DP studies.
In
particular, scale up the reported HA synthesis method is being explored
so that
an adequate amount of enhanced powder may be produced to use in the 3DP
process. If this is achieved, it will enable a new dimension of
tailoring but
while there are separate chemical and physical challenges, some of
which are
discussed here, there are certainly still many more difficulties to be
unearthed in an attempt to catch up with evolution’s efforts. In
conclusion,
the research presented highlights the importance of multidisciplinary
collaborations
within the biomedical field, which may enable the next generation of
customised
implants to be created.
Acknowledgements
The
author would like to
acknowledge the financial support of the Chancellor’s Scholarship
and the
Institute of Advanced Study both at the University of Warwick. Dr Kajal
K. Mallick,
who is sadly no longer with us, is also acknowledged for his
involvement in the
reported research.
References
Boanini, E., M. Gazzano and
A. Bigi (2010).
"Ionic substitutions in calcium phosphates synthesized at low
temperature." Acta Biomaterialia 6(6):
1882-1894.
Bohner,
M. (2009). "Silicon-substituted calcium phosphates–a critical
view."
Biomaterials 30(32): 6403-6406.
Bose, S. and
S. K. Saha (2003). "Synthesis and characterization of hydroxyapatite
nanopowders by emulsion technique." Chemistry of Materials 15(23):
4464-4469.
Bose,
S., S. Vahabzadeh and A. Bandyopadhyay (2013). "Bone tissue engineering
using 3D printing." Materials Today 16(12):
496-504.
Butscher,
A., M. Bohner, C. Roth, A. Ernstberger, R. Heuberger, N. Doebelin, P.
R. von
Rohr and R. Muller (2012). "Printability of calcium phosphate powders
for
three-dimensional printing of tissue engineering scaffolds." Acta
Biomaterialia 8(1): 373-385.
Capuccini,
C., P. Torricelli, F. Sima, E. Boanini, C. Ristoscu, B. Bracci, G.
Socol, M.
Fini, I. Mihailescu and A. Bigi (2008). "Strontium-substituted
hydroxyapatite coatings synthesized by pulsed-laser deposition: in
vitro
osteoblast and osteoclast response." Acta Biomaterialia 4(6):
1885-1893.
Cazalbou,
S., C. Combes, D. Eichert and C. Rey (2004). "Adaptative
physico-chemistry
of bio-related calcium phosphates." Journal of Materials Chemistry 14(14): 2148-2153.
Cengiz,
B., Y. Gokce, N. Yildiz, Z. Aktas and A. Calimli (2008). "Synthesis and
characterization of hydroxyapatite nanoparticles." Colloids and
Surfaces
a-Physicochemical and Engineering Aspects 322(1-3):
29-33.
Chamay,
A. and P. Tschantz (1972). "Mechanical Influences in Bone Remodeling -
Experimental Research on Wolffs Law." Journal of Biomechanics 5(2): 173-&.
Cox,
S. C. (2013). Synthesis and 3D printing of hydroxyapatite scaffolds for
applications in bone tissue engineering, University of Warwick.
Cox, S. C.,
P. Jamshidi, L. M. Grover and K. K. Mallick (2014). "Low temperature
aqueous precipitation of needle-like nanophase hydroxyapatite." Journal
of
Materials Science: Materials in Medicine 25(1):
37-46.
Cox,
S. C., P. Jamshidi, L. M. Grover and K. K. Mallick (2014). "Preparation
and characterisation of nanophase Sr, Mg, and Zn substituted
hydroxyapatite by
aqueous precipitation." Materials Science and Engineering: C 35: 106-114.
Cox,
S. C., R. I. Walton and K. K. Mallick (2014). "Comparison of Techniques
for the Synthesis of Hydroxyapatite."
Drouet,
C. (2013). "Apatite formation: why it may not work as planned, and how
to
conclusively identify apatite compounds." BioMed research international
2013.
Elliott,
J. C. (1994). Structure and chemistry of the apatites and other calcium
orthophosphates, Elsevier Amsterdam.
Gibson,
L. J. (1985). "The mechanical behaviour of cancellous bone." Journal
of Biomechanics 18(5): 317-328.
Hollister,
S. J. (2005). "Porous scaffold design for tissue engineering." Nature
Materials 4(7): 518-524.
Hutmacher,
D. W. (2000). "Scaffolds in tissue engineering bone and cartilage."
Biomaterials 21(24): 2529-2543.
Khalyfa,
A., S. Vogt, J. Weisser, G. Grimm, A. Rechtenbach, W. Meyer and M.
Schnabelrauch (2007). "Development of a new calcium phosphate
powder-binder system for the 3D printing of patient specific implants."
Journal of Materials Science-Materials in Medicine 18(5):
909-916.
Landi,
E., G. Logroscino, L. Proietti, A. Tampieri, M. Sandri and S. Sprio
(2008).
"Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo
behaviour." Journal of Materials Science: Materials in Medicine 19(1): 239-247.
Landi,
E., G. Logroscino, L. Proietti, A. Tampieri, M. Sandri and S. Sprio
(2008).
"Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo
behaviour." Journal of Materials Science-Materials in Medicine 19(1): 239-247.
Leong,
K. F., C. M. Cheah and C. K. Chua (2003). "Solid freeform fabrication
of
three-dimensional scaffolds for engineering replacement tissues and
organs." Biomaterials 24(13):
2363-2378.
Leventouri,
T. (2006). "Synthetic and biological hydroxyapatites: Crystal structure
questions." Biomaterials 27(18):
3339-3342.
Macchetta,
A., I. G. Turner and C. R. Bowen (2009). "Fabrication of HA/TCP
scaffolds
with a graded and porous structure using a camphene-based
freeze-casting
method." Acta Biomaterialia 5(4):
1319-1327.
Narasaraju,
T. S. B. and D. E. Phebe (1996). "Some physico-chemical aspects of
hydroxylapatite." Journal of Materials Science 31(1):
1-21.
Phillips,
M. J., J. A. Darr, Z. B. Luklinska and I. Rehman (2003). "Synthesis and
characterization of nano-biomaterials with potential osteological
applications." Journal of Materials Science-Materials in Medicine 14(10): 875-882.
Posner,
A. S. (1969). "Crystal chemistry of bone mineral." Physiol Rev 49(4): 760-792.
Raynaud,
S., E. Champion, D. Bernache-Assollant and P. Thomas (2002). "Calcium
phosphate apatites with variable Ca/P atomic ratio I. Synthesis,
characterisation and thermal stability of powders." Biomaterials 23(4): 1065-1072.
Ritchie,
R. O., J. W. Ager and G. Balooch (2006). "Fracture, aging, and disease
in
bone." Journal of Materials Research 21(8):
1878-1892.
Roy,
T. D., J. L. Simon, J. L. Ricci, E. D. Rekow, V. P. Thompson and J. R.
Parsons
(2003). "Performance of degradable composite bone repair products made
via
three-dimensional fabrication techniques." Journal of Biomedical
Materials
Research Part A 66A(2): 283-291.
Roy,
T. D., J. L. Simon, J. L. Ricci, E. D. Rekow, V. P. Thompson and J. R.
Parsons
(2003). "Performance of hydroxyapatite bone repair scaffolds created
via
three-dimensional fabrication techniques." Journal of Biomedical
Materials
Research Part A 67A(4): 1228-1237.
Sachs,
E., M. Cima, J. Cornie, D. Brancazio, J. Bredt, A. Curodeau, T. Fan, S.
Khanuja, A. Lauder, J. Lee and S. Michaels (1993). "Three-Dimensional
Printing: The Physics and Implications of Additive Manufacturing." CIRP
Annals - Manufacturing Technology 42(1):
257-260.
Sanchez-Salcedo,
S., D. Arcos and M. Vallet-Regi (2008). "Upgrading calcium phosphate
scaffolds for tissue engineering applications." Progress in Bioceramics
377: 19-42.
Schmitz,
J. P. and J. O. Hollinger (1986). "The Critical Size Defect as an
Experimental-Model for Craniomandibulofacial Nonunions." Clinical
Orthopaedics and Related Research(205): 299-308.
Suchanek,
W. and M. Yoshimura (1998). "Processing and properties of
hydroxyapatite-based biomaterials for use as hard tissue replacement
implants." Journal of Materials Research 13(1): 94-117.
Travitzky,
N., A. Bonet, B. Dermeik, T. Fey, I. Filbert‐Demut,
L. Schlier, T. Schlordt and P. Greil (2014). "Additive Manufacturing of
Ceramic‐Based Materials." Advanced
Engineering Materials.
Webster,
T. J., C. Ergun, R. H. Doremus, R. W. Siegel and R. Bizios (2001).
"Enhanced osteoclast-like cell functions on nanophase ceramics."
Biomaterials 22(11): 1327-1333.
Weiner,
S. and H. D. Wagner (1998). "The material bone: Structure mechanical
function relations." Annual Review of Materials Science 28:
271-298.
Xue,
W., J. L. Moore, H. L. Hosick, S. Bose, A. Bandyopadhyay, W. Lu, K.
Cheung and
K. D. Luk (2006). "Osteoprecursor cell response to strontium‐containing
hydroxyapatite ceramics." Journal of Biomedical Materials Research Part
A 79(4): 804-814.
You,
L. D., J. H. Chen, C. Liu and C. A. Simmons (2010). "Boning up on
Wolff's
Law: Mechanical regulation of the cells that make and maintain bone."
Journal of Biomechanics 43(1):
108-118.