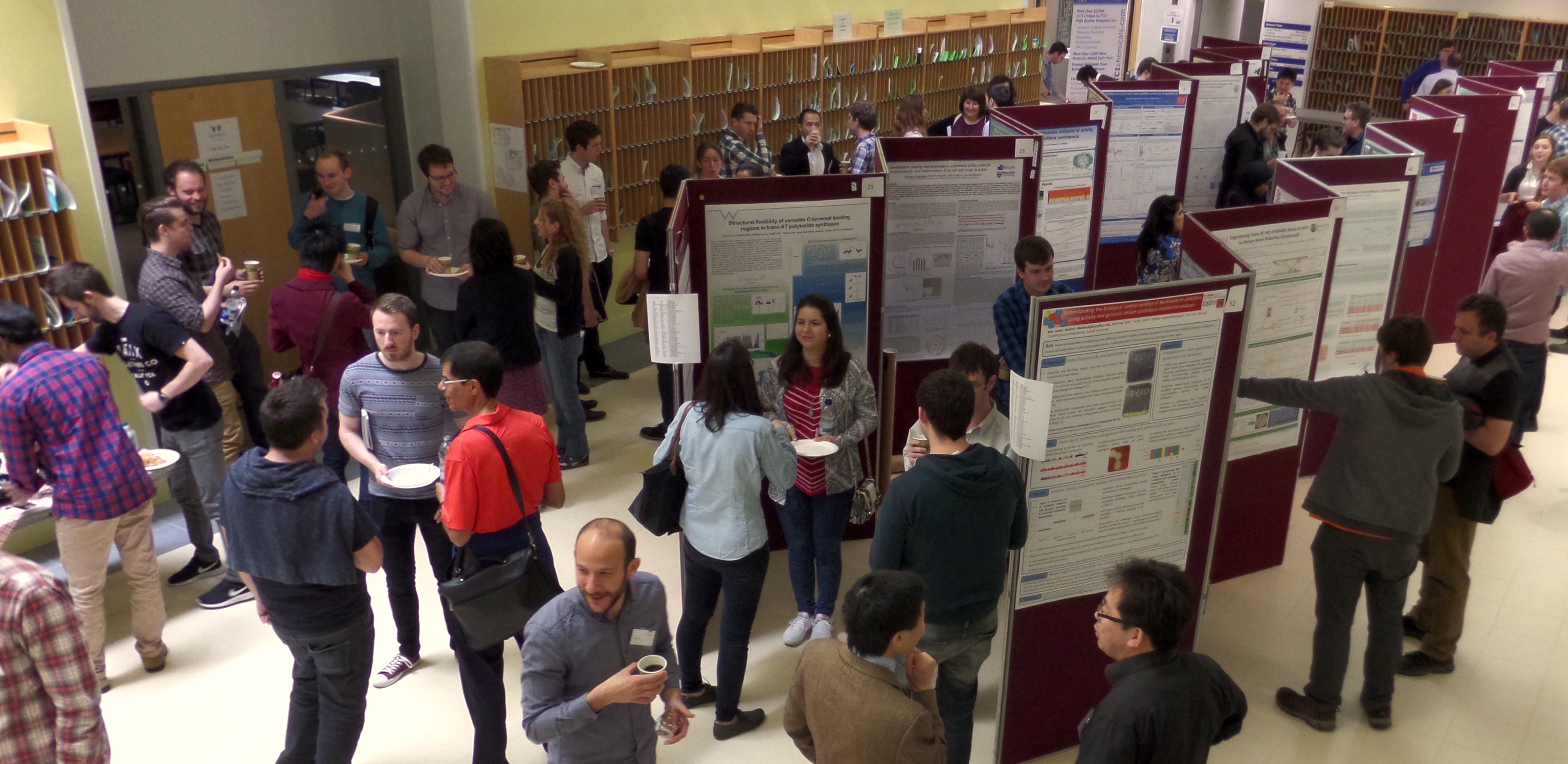
Current Trends in Natural Products Research from the CBNP10 Symposium at Warwick
DOI:
https://doi.org/10.31273/eirj.v4i1.154Keywords:
Natural Products, Biosynthesis, Antibiotics.Abstract
Natural products are compounds that are produced by living organisms. They have numerous applications in our everyday life, from antibiotics to herbicides. They possess great chemical and structural diversity, which gives them a leading position as a source of new drugs. Many institutions worldwide are focusing more and more on natural product research, with microorganisms and plants being the most common source for discovery of new compounds. On the 30th June and 1st July 2016, early-career scientists working in the field of natural products gathered at the University of Warwick for the 10th edition of the Chemistry and Biology of Natural Products Symposium (CBNP10). This critical reflection reviews, in the context of the current research in the field, the major considerations that arose from this meeting.Downloads
References
Anderson R. C. G. R., H. M. Higgins Jr and C. D. Pettinga (1961), “Symposium: how a drug is born.”, Cincinnati Journal of Medicine, 42, 49–60.
Baltz R. H. (2008), “Renaissance in antibacterial discovery from actinomycetes.”, Current Opinion in Pharmacology, 8, (5), 557-63.
Booth, T. J., A. Silke, R. J. Capon and B. Wilkinson (2016), “Synchronous intramolecular cycloadditions of the polyene macrolactam polyketide heronamide C.”, Chemical Communications, 52, 6383-86.
Crone W. J. K., N. M. Vior, J. Santos-Aberturas, L. G. Schmitz, F. J. Leeper and A. W. Truman (2016), “Dissecting Bottromycin Biosynthesis Using Comparative Untargeted Metabolomics.”, Angewandte Chemie International Edition, 55, (33), 9639-43.
Fayed B., D. A. Ashford, A. M. Hashem, M. A. Amin, O. N. El Gazayerly, M. A. Gregory and M. C.M. Smith (2015), “Multiplexed integrating plasmids for engineering the erythromycin gene cluster for expression in Streptomyces and combinatorial biosynthesis.”, Applied and Environmental Microbiology, 81, (23), 1-36.
Khaldi N., F. T. Seifuddin, G. Turner, D. Haft, W. C. Nierman, K. H. Wolfe and N. D. Fedorova (2010), “SMURF: Genomic mapping of fungal secondary metabolite clusters.”, Fungal Genetics and Biology, 47, 736-41.
Littleson M.M., C. J. Russell, E. C. Frye, K. B. Ling, C. Jamieson and J. B. Watson (2016), “Synthetic Approaches to Coronafacic Acid, Coronamic Acid, and Coronatine.” Synthesis, 48, (20), 3429-48.
Medema M. H., and M. A. Fischbach (2015), “Computational approaches to natural product discovery.”, Nature Chemical Biology, 11, (9), 639-48.
Parascandolo J.S., J. Havemann, H. K. Potter, F. Huang, E. Riva, J. Connolly, I. Wilkening, L. Song, P. F. Leadlay and M. Tosin (2016), “Insights into 6-Methylsalicylic Acid Bio-assembly by Using Chemical Probes.”, Angewandte Chemie International Edition, 55, (10), 3463-67.
Shen, B. (2015), “A new golden age of natural products drug discovery.”, Cell, 153, 1297-300.
Stadler M., and D. Hoffmeister (2015), “Fungal natural products—the mushroom perspective.”, Frontiers in Microbiology, 6, 127.
Umezawa, H. (1958), “Kanamycin: Its Discovery.” Annals of the New York Academy of Sciences, 76, 20–26.
Weber T., K. Blin, S. Duddela, D. Krug, H.U. Kim, R. Bruccoleri, S. Y. Lee, M. A. Fischbach, R. Müller, W. Wohlleben, R. Breitling, E. Takano and M. H. Medema (2015), “antiSMASH 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters.”, Nucleic Acids Research, 43, 237-43.
Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC-BY), which permits use and redistribution of the work provided that the original author and source are credited, a link to the license is included, and an indication of changes which were made. Third-party users may not apply legal terms or technological measures to the published article which legally restrict others from doing anything the license permits.
If accepted for publication authors’ work will be made open access and distributed under a Creative Commons Attribution (CC-BY) license unless previously agreed with Exchanges’ Editor-in-Chief prior to submission.
Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work. (see: The Effect of Open Access)
